My understanding is that it is typically unusual for a drugs to fail in phase 3 trials and that is exactly what happened to the recent ALS drug, RELYVRIO.
Starting this thread to initiate a retrospective discussion on what important takeaways from this case for the community.
An advisory committee discussed phase 2 results in 2022 before RELYVRIO was conditionally approved. Interesting to see that FDA statisticians thought the results were not as compelling.
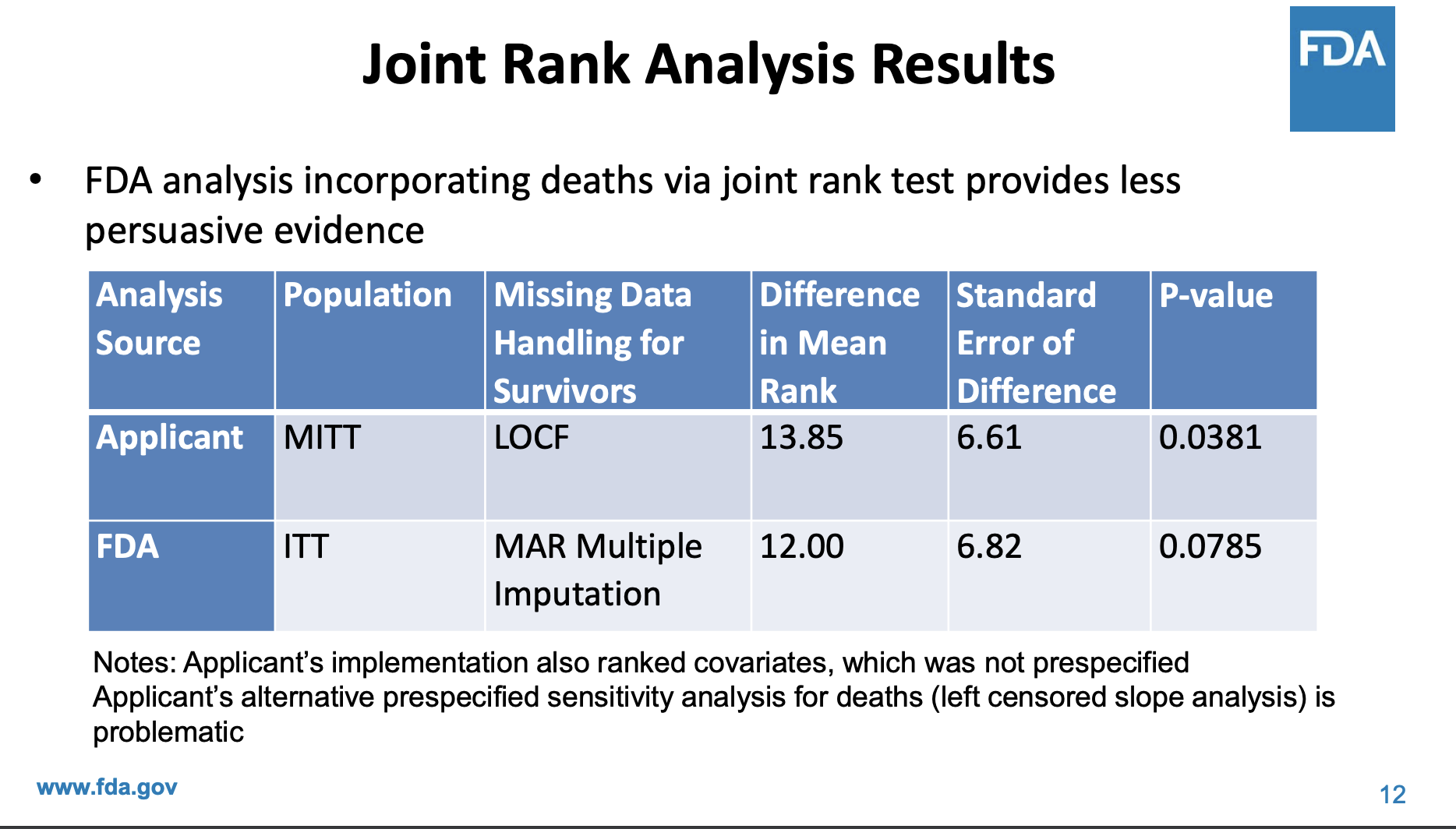
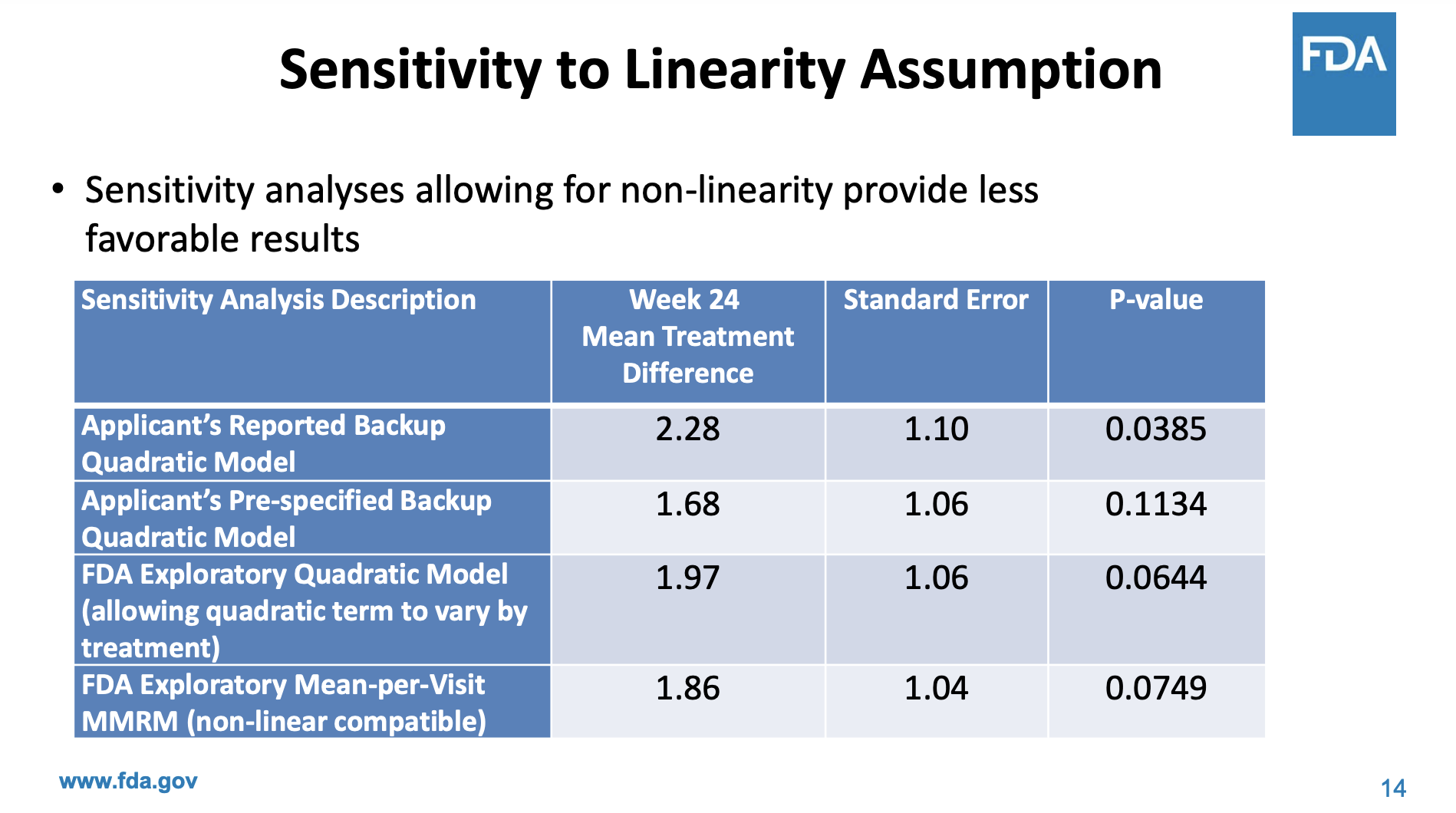
In the end, the results were borderline in the initial presentation and the majority of the advisory committee voted to no. Then later, based on additional exploratory evidence, the majority voted yes. Personally, I think a conditional approval subject to a better trial was the right call from a risk-benefit tradeoff.
Meeting materials from March, 2022.
Meeting materials from September, 2022