AD Wagner, NL Syn, M Moehler, W Grothe, WP Yong, BC Tai, J Ho and S Unverzagt,
The Cochrane database of systematic reviews, 29 2017 08
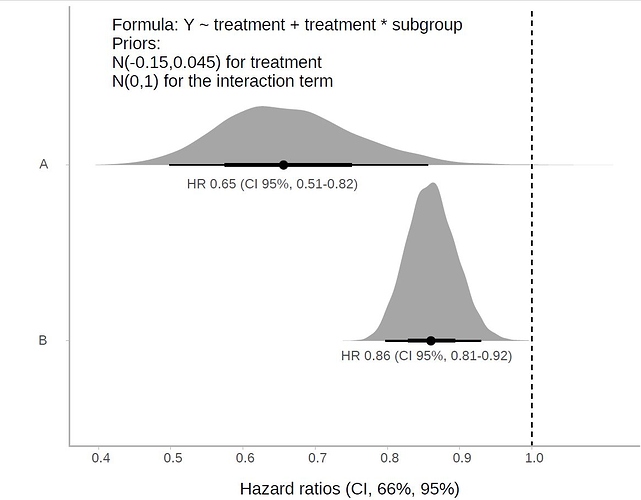
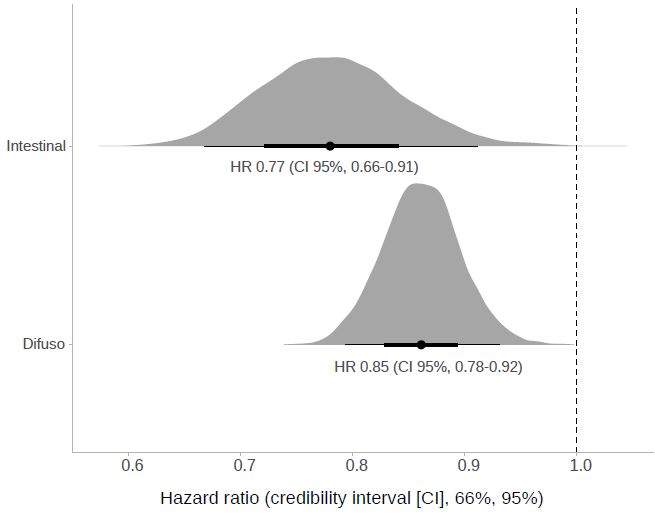
Gastric cancer is the fifth most common cancer worldwide. In "Western" countries, most people are either diagnosed at an advanced stage, or develop a relapse after surgery with curative intent. In people with advanced disease, significant benefits from targeted therapies are currently limited to HER-2 positive disease treated with trastuzumab, in combination with chemotherapy, in first-line. In second-line, ramucirumab, alone or in combination with paclitaxel, demonstrated significant survival benefits. Thus, systemic chemotherapy remains the mainstay of treatment for advanced gastric cancer. Uncertainty remains regarding the choice of the regimen.To assess the efficacy of chemotherapy versus best supportive care (BSC), combination versus single-agent chemotherapy and different chemotherapy combinations in advanced gastric cancer.We searched the Cochrane Central Register of Controlled Trials, MEDLINE and Embase up to June 2016, reference lists of studies, and contacted pharmaceutical companies and experts to identify randomised controlled trials (RCTs).We considered only RCTs on systemic, intravenous or oral chemotherapy versus BSC, combination versus single-agent chemotherapy and different chemotherapy regimens in advanced gastric cancer.Two review authors independently identified studies and extracted data. A third investigator was consulted in case of disagreements. We contacted study authors to obtain missing information.We included 64 RCTs, of which 60 RCTs (11,698 participants) provided data for the meta-analysis of overall survival. We found chemotherapy extends overall survival (OS) by approximately 6.7 months more than BSC (hazard ratio (HR) 0.3, 95% confidence intervals (CI) 0.24 to 0.55, 184 participants, three studies, moderate-quality evidence). Combination chemotherapy extends OS slightly (by an additional month) versus single-agent chemotherapy (HR 0.84, 95% CI 0.79 to 0.89, 4447 participants, 23 studies, moderate-quality evidence), which is partly counterbalanced by increased toxicity. The benefit of epirubicin in three-drug combinations, in which cisplatin is replaced by oxaliplatin and 5-FU is replaced by capecitabine is unknown.Irinotecan extends OS slightly (by an additional 1.6 months) versus non-irinotecan-containing regimens (HR 0.87, 95% CI 0.80 to 0.95, 2135 participants, 10 studies, high-quality evidence).Docetaxel extends OS slightly (just over one month) compared to non-docetaxel-containing regimens (HR 0.86, 95% CI 0.78 to 0.95, 2001 participants, eight studies, high-quality evidence). However, due to subgroup analyses, we are uncertain whether docetaxel-containing combinations (docetaxel added to a single-agent or two-drug combination) extends OS due to moderate-quality evidence (HR 0.80, 95% CI 0.71 to 0.91, 1466 participants, four studies, moderate-quality evidence). When another chemotherapy was replaced by docetaxel, there is probably little or no difference in OS (HR 1.05; 0.87 to 1.27, 479 participants, three studies, moderate-quality evidence). We found there is probably little or no difference in OS when comparing capecitabine versus 5-FU-containing regimens (HR 0.94, 95% CI 0.79 to 1.11, 732 participants, five studies, moderate-quality evidence) .Oxaliplatin may extend (by less than one month) OS versus cisplatin-containing regimens (HR 0.81, 95% CI 0.67 to 0.98, 1105 participants, five studies, low-quality evidence). We are uncertain whether taxane-platinum combinations with (versus without) fluoropyrimidines extend OS due to very low-quality evidence (HR 0.86, 95% CI 0.71 to 1.06, 482 participants, three studies, very low-quality evidence). S-1 regimens improve OS slightly (by less than an additional month) versus 5-FU-containing regimens (HR 0.91, 95% CI 0.83 to 1.00, 1793 participants, four studies, high-quality evidence), however since S-1 is used in different doses and schedules between Asian and non-Asian population, the applicability of this finding to individual populations is uncertain.Chemotherapy improves survival (by an additional 6.7 months) in comparison to BSC, and combination chemotherapy improves survival (by an additional month) compared to single-agent 5-FU. Testing all patients for HER-2 status may help to identify patients with HER-2-positive tumours, for whom, in the absence of contraindications, trastuzumab in combination with capecitabine or 5-FU in combination with cisplatin has been shown to be beneficial. For HER-2 negative people, all different two-and three-drug combinations including irinotecan, docetaxel, oxaliplatin or oral 5-FU prodrugs are valid treatment options for advanced gastric cancer, and consideration of the side effects of each regimen is essential in the treatment decision. Irinotecan-containing combinations and docetaxel-containing combinations (in which docetaxel was added to a single-agent or two-drug (platinum/5-FUcombination) show significant survival benefits in the comparisons studied above. Furthermore, docetaxel-containing three-drug regimens have increased response rates, but the advantages of the docetaxel-containing three-drug combinations (DCF, FLO-T) are counterbalanced by increased toxicity. Additionally, oxaliplatin-containing regimens demonstrated a benefit in OS as compared to the same regimen containing cisplatin, and there is a modest survival improvement of S-1 compared to 5-FU-containing regimens.Whether the survival benefit for three-drug combinations including cisplatin, 5-FU, and epirubicin as compared to the same regimen without epirubicin is still valid when second-line therapy is routinely administered and when cisplatin is replaced by oxaliplatin and 5-FU by capecitabine is questionable. Furthermore, the magnitude of the observed survival benefits for the three-drug regimens is not large enough to be clinically meaningful as defined recently by the American Society for Clinical Oncology (Ellis 2014). In contrast to the comparisons in which a survival benefit was observed by adding a third drug to a two-drug regimen at the cost of increased toxicity, the comparison of regimens in which another chemotherapy was replaced by irinotecan was associated with a survival benefit (of borderline statistical significance), but without increased toxicity. For this reason irinotecan/5-FU-containing combinations are an attractive option for first-line treatment. Although they need to be interpreted with caution, subgroup analyses of one study suggest that elderly people have a greater benefit form oxaliplatin, as compared to cisplatin-based regimens, and that people with locally advanced disease or younger than 65 years might benefit more from a three-drug regimen including 5-FU, docetaxel, and oxaliplatin as compared to a two-drug combination of 5-FU and oxaliplatin, a hypothesis that needs further confirmation. For people with good performance status, the benefit of second-line chemotherapy has been established in several RCTs.