We are planning a study to evaluate the impact of diabetes on acute toxicities experienced by patients with cervical cancer who are receiving concurrent chemotherapy and radiotherapy. Acute toxicities refer to toxicities that occur during treatment. Clinically this is an important issue as patients with severe acute toxicity often end up receiving less treatment or breaks in treatment.
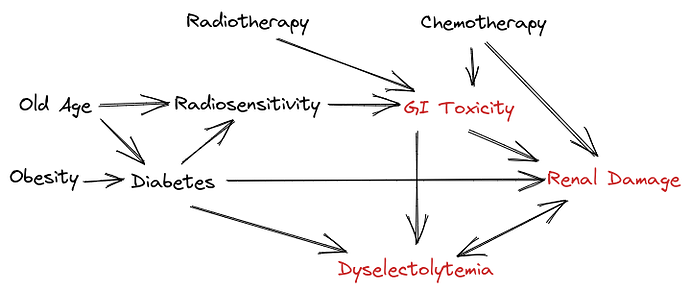
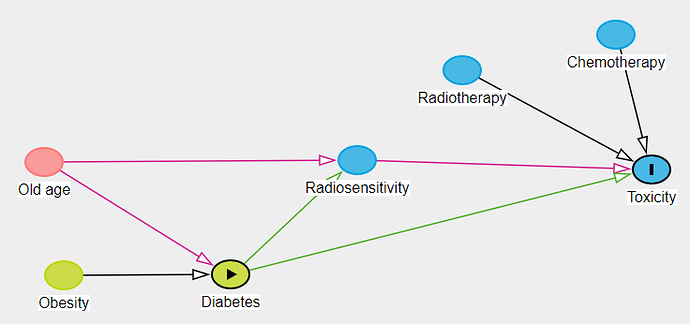
In preparation for the analysis of the study we have prepared a DAG which is shown here in the image (prepared in excalidraw hence not exactly a proper diagram)
To explain the relationships:
- Old age and obesity increase the risk of having a diabetes
- Patients who are older may have a poorer tolerance to radiotherapy and chemotherapy (evidence is modest) which leads to increased risk of GI toxicity
- Radiotherapy and chemotherapy together can cause GI Toxicity
- Chemotherapy can cause Renal Damage.
- Renal damage results in dyselectrolytemia and some forms of dyselectrolytemia result in further exacerbation of renal damage.
- GI Toxicity often results in renal damage due to dehydration resulting from diarrhea and vomiting. This can also cause dyselectrolytemia.
- Diabetes causes malnutrition and inflammation and these states may also be associated with increased radio-sensitivity and thereby worse radiation toxicities
Note that in the diagram the radio-sensitivity link is to GI Toxicity and not directly to renal damage as during radiotherapy we ensure a very low dose of radiation to kidney which is associated with a low clinically relevant renal toxicity during the treatment.
They study we are planning is a retrospective cross sectional study of patients treated for cervical cancer. The link to the registered study protocol in OSF is OSF. While the primary endpoint is the prevalance of toxicities in the patients with diabetes, we would also like to ascertain the association using a multivariable regression model to obtain adjusted estimates.
We need advice regarding the appropriate analytical strategy to use so that we can get reasonably reliable estimates given the complex relationships (as an aside we are selecting patients treated with a homogenous treatment protocol where radiotherapy is delivered using a single technique to same dose levels).
Current Strategy:
We are planning to develop two regression models where acute toxicity (the dependant variable) will be evaluated. Method one will use a maximum grade method and use a ordinal regression model. Method two will use a toxicity index (weighted sum of ordered toxicity scores) and will also use ordinal regression.
The following independent variables are planned to be included in the model:
- Age in years
- Height in cm
- Weigh in kg
- Baseline serum albumin (marker of nutrition)
- Baseline serum hemoglobin (anemia can be nutritional and associated with chronic disease).
- Baseline total leucocyte count (marker of nutrition and immune function)
- Baseline serum creatinine (marker of baseline renal function)
- Baseline neutrophil count (as with leucocyte count marker of nutrition and immune function)
- Volume of the plannig target (The high dose radiation volume)
- Use of simultaneous integrated boost (yes / no)
- Comorbidity
Comorbidity will be coded as follows:
- No comorbidity
- DM only
- DM + Other comorbidity
- Other comorbidity without DM
As old age is associated with diabetes we have planned an interaction term with age and comorbidity. Continous variables like age, height, weight, serum albumin, hemogllobin etc will be modelled with restricted cubic splines.
The dependant variable - toxicity will be modelled as an ordinal variable to retain the maxium information and we will develop models for toxicity as a cumulative as well seperately for toxicities which belong to families:
- GI : Diarrhea, nausea, vomiting
- Dyselectrolytemia : Various electrolyte abnormalities
- Hematological toxicities
- Infections
We estimate we will have around 350 - 400 patients available for analysis. Given the nature of treatment, almost everyone is expected to have some degree of toxicity with approximately 10 - 20% having severe toxicity (which will reflect in higher scores).
Note that this study would be interesting as India is currently the diabetes capital of the world and we estimate that about 25 - 30% of our patients have diabetes !