Seeking comment on the methods used in this study, where the authors conclude as shown in bulleted key points below.
As a patient advocate I am not qualified to critique the methods or conclusion but have read other articles (I will post later) indicating that fractional doses of CAR t-cells over 3 days can reduce the risk of CRS (cytokine release syndrome) and that use of steroids to treat CRS does limit the efficacy of CAR tell therapy. This concern is also based on the known inhibitory effect of steroids on immune cells (they are used to treat lymphoma).
My reading of it is that this was not a controlled study and the size of it was very small. My concern is that the acceptance of it as a convincing finding is limiting the study of alternative dosing schedules of CAR t-cells that could improve both patient safety (literally save lives) and improve the efficacy of the treatment.
-
Early intervention with tocilizumab and steroids does not impact the expansion or persistence of SCRI-CAR19v1 CAR T cells in B-ALL.
-
Administration of tocilizumab and corticosteroids does not negatively impact the efficacy of SCRI-CAR19v1.
Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy
https://ashpublications.org/blood/article/134/24/2149/381162/Preemptive-mitigation-of-CD19-CAR-T-cell-cytokine
1 Like
Thank you for highlighting this issue, Karl. It looks to me like a classic absence-of-evidence fallacy. To promote wider discussion, I cut & paste a relevant section of the paper below:
LFS = Leukemia-Free Survival
MRD = minimal residual disease
‘DLT’ cohort ≈ usual care for CRS
EI = Early-Intervention against CRS
CRS = cytokine release syndrome, generally thought to be an on-target toxicity of anti-cancer therapy
Dosing of tocilizumab and/or corticosteroids is not associated with increased incidence of serious infection
There is a possibility that the use of immunomodulatory or immunosuppressive medications could result in an increased infection risk. There was no difference in the rate of infection post-CAR T-cell infusion when comparing subjects who did or did not receive steroids (P = .247). Tocilizumab is known to cause neutropenia; however, in subjects who did and did not receive tocilizumab, there was no difference in the percentage of those whose absolute neutrophil count recovered to >200 per microliter by 21 days from CAR T-cell infusion (P = .206; relative risk, 0.76).
Early use of tocilizumab and corticosteroids does not decrease the efficacy of SCRI-CAR19v1
There was no impact of EI with tocilizumab or dexamethasone on the MRD-negative CR rate (91% in the DLT cohort vs 95% in the EI cohort). The durability of the remission also was not impacted by the CRS intervention strategy, with overlapping LFS and OS between the DLT and EI cohorts (Figure 5A-B). When segregated into groups based on the immunomodulatory intervention received (no intervention, tocilizumab only, and steroids with or without tocilizumab), no statistical difference was noted for LFS or OS (Figure 5C-D).
Figure 5.
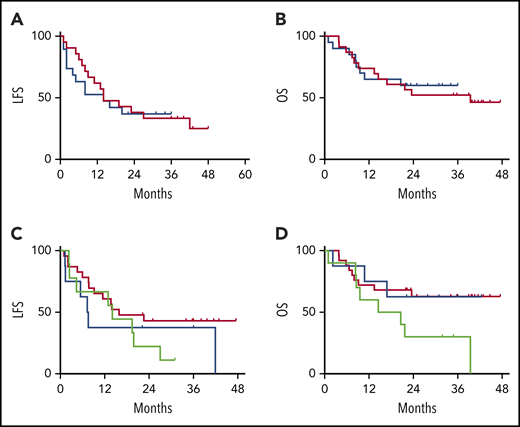
VIEW LARGEDOWNLOAD PPT
LFS and OS are unaffected by CRS treatment strategy or administered CRS-directed treatment. All subjects were analyzed for LFS and OS following CAR T-cell infusions. The DLT cohort (red) and the EI cohort (blue) had similar LFS Kaplan-Meier curves (A) and OS Kaplan-Meier curves (B). Separately, all subjects were categorized per the specific intervention received. Intervention groups are steroid with or without tocilizumab (green), tocilizumab (blue), and none (red), with no difference between LFS Kaplan-Meier curves (C) and OS Kaplan-Meier curves (D).
2 Likes
Thank you for supplementing my inquiry, David.
Here is the competing approach to to giving CAR t-cells that suggests that fractionating the dose improves safety, limits need for steroids and dose reductions, and potentially improves efficacy.
Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia
PURPOSE
The anti-CD19 chimeric antigen receptor T-cell therapy tisagenlecleucel (CTL019) has an 81% response rate in children with relapsed or chemotherapy refractory (r/r) B-cell acute lymphoblastic leukemia (ALL). Cytokine release syndrome (CRS) is a life-threatening treatment-related toxicity that limits the full therapeutic potential in adults. We report outcomes for adults with r/r ALL treated with an optimized CTL019 dosing and CRS management strategy.
METHODS
Adults with r/r B-cell ALL received CTL019 in 1 of 2 trials. Patients received lymphodepletion followed by CTL019 as either a one-time infusion or fractionated infusions split over 3 days (day 1, 10%; day 2, 30%; day 3, 60%), which allowed for day 2 and day 3 doses to be held for early CRS. Total planned CTL019 dose varied with adaptive protocol modifications in response to efficacy and CRS toxicity.
RESULTS
Thirty-five adults with r/r ALL received CTL019 in 1 of 3 dosing cohorts. The low-dose cohort (n = 9) received single or fractionated dosing and had manageable toxicity with a 33% complete remission (CR) rate. In the high-dose single infusion cohort, 3 of 6 patients with refractory CRS concurrent with culture-positive sepsis died, and 3 achieved CR. The 20 patients in the high-dose fractionated (HDF) cohort had a 90% CR rate and manageable CRS. The HDF cohort had the highest survival, with a 2-year overall survival of 73% (95% CI, 46% to 88%) and event-free survival of 49.5% (95% CI, 21% to 73%).
CONCLUSION
Fractionated dosing of CTL019 with intrapatient dose modification optimizes safety without compromising efficacy in adults with r/r ALL.
2 Likes
