I will do that here to provide an example of the state of the dogma. Here I describe, in lay terms (very basic, forgive me) the evolution of the “science” of adult (later called “acute”) respiratory distress syndrome (ARDS).
The Origin of Adult Respiratory Distress Syndrome (ARDS)
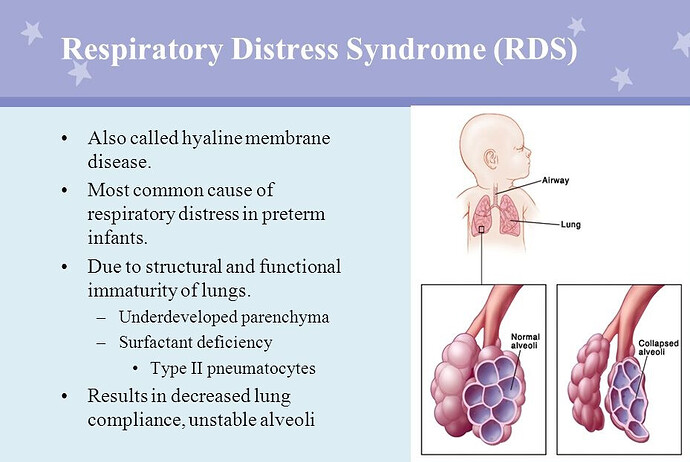
In the 60s “Respiratory Distress Syndrome” (RDS) of premature newborns was common.(eg death a child of President John Kennedy). This was caused by lack of a biochemical called “surfactant” which functioned to reduced severe and intractable collapse of the tiny lung air sacs (alveoli). The lack of surfactant produced RDS.
In RDS (hyaline membrane disease), the alveoli become progressively more difficult to inflate even with a mechanical ventilator. The lungs would become stiffer (harder to inflate) and the “chest Xray” (CXR) would show fluid diffusely in both the lungs. Blood oxygen would fall to severe levels despite high oxygen administration. in other words the partial pressure of oxygen (PaO2) and the saturation of arterial hemoglobin with Oxygen by pulse oximetry (SPO2) would fall in relation to the fraction of inspired oxygen (FIO2) producing a low Pao2/FIO2 or SPO2/FIO2.
1st Idea: Tom Petty suggests adults can also have “Hyaline Membrane Disease” (RDS)
In the late 60s, a pulmonary physician, Thomas Petty noted that adults had a similar “appearing” condition (to RDS of the newborn) with stiffening lungs and CXR findings after viral pneumonia, major trauma, pancreatitis, the inhalation of vomit, and many other diverse conditions. He theorized that this was also “hyaline membrane disease” and that it had a similar cause as RDS of newborns and he called the condition Adult Respiratory Distress Syndrome (ARDS). Indeed these patients had strikingly similar presentation and progression with increasing lung stiffening with increasing pressure required for ventilation.
Even in severe cases the lung pathology was often not the cause of death. Rather it was often not possible to wean them from the ventilator and they commonly died of “recovery failure” with secondary infection or by patient or family request. Mortality was highly variable but often quoted in the mid 30s to 45% range for more severe cases.
Clinical trials of “ARDS” begin lumping the causal diseases together thinking they were treating a single disease of the lung
Consensus groups guessed broad threshold set measurements for ARDS for RCT and, under these guessed measurements, the trials lumped ALL the diverse diseases inducing the appearance of RDS into Petty’s ARDS. However, surfactant treatment did not provide benefit.
All drug trials for Petty’s ARDS fail
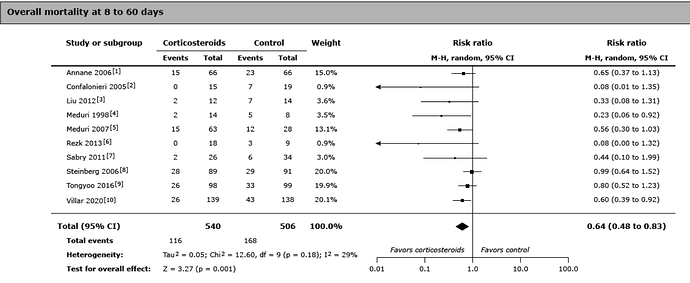
None of the drugs tried over many decades worked for Dr. Petty’s lumped diseases, although corticosteroids seemed to work or were harmful in various trials.
2nd Idea: “Recovery failure is due to ventilator induced lung injury (VILI)”
In the late 1990s, given the lack of success, a new idea developed that it was the ventilator induced inflation pressure injury causing failure to recover. A trial showed the 6cc/ Kg breath from the ventilator was better than 12cc/kg. This led to the low volume ventilation but the gain in outcome was quite modest and unfortunately the 12cc/kg used in the trial was a high ventilator volume not normally prescribed (usual volume was 10cc/kg).
3rd idea: Recovery failure is due to patients “fighting” the new low volume settings of the ventilator
As noted, improvement with low ventilation volumes was quite modest but one problem with low volumes was that patients often felt like they were suffocating. This air hunger (Tobin called it waterboarding) caused considerable suffering and higher sedation (which was know to cause adverse effect on weaning) was required to prevent wide pressure swings leading to lung injury. The solution was thought to be paralysis of the patients. However, paralysis trials were performed which did not improve survival.
4th idea: Patient Self-Inflicted Lung Injury" (P-SILI) is suspected as the cause of recovery failure.
The next the idea was that the patient herself was causing the lung injury before being connected to a ventilator by hard breathing. This was called “P-SILI”. This led to very early intubation and mechanical ventilation to minimize the effort. This aggressive treatment idea (based on a theory) failed during the COVID pandemic and produced much controversy.
In summary.
Tom Petty’s 1960s idea was an example of the wrongful (in retrospect) application of parsimony, the view that the simplest explanation is often the correct one. It was a simpler time. DNA had only recently been discovered. Disease was perceived in simpler terms. Seeking a common causal basis for both RDS of the new born and the similar appearing pulmonary dysfunction associated with adult diseases was logical.
RDS of the newborn had been successfully treated. Not so for Petty’s idea of “ARDS”. However, buoyed by the success of RDS treatment and believing Petty was right, optimistic researchers over 5 decades cycled through drugs, ventilator adjustments, paralysis, and early intubation seeking a unified treatment for Petty’s perceived but elusive “common cause” of recovery failure. After 37 years, by the turn of the 21st century Petty’s lumping idea had become dogma, in all the textbooks and taught worldwide. The RCTs of the 21st century embraced Petty’s idea which had now become fully entrenched dogma.
The pandemic exposes the paradigm
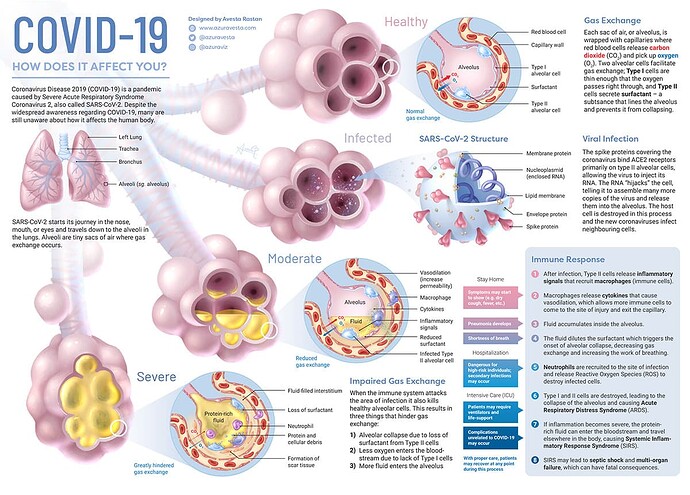
Then the pandemic showed that parsimony (lumping) had failed and exposed the extreme to which the dogma combining such disparate diseases had evolved over 56 years. Severe COVID pneumonia, which includes actual infection and destruction of type II alveolar cells, was so profoundly different than RDS of the newborn and many other causes of “ARDS” yet it was just summarily lumped in with all the other diseases because it met the guessed measurements and Tom Petty included viral pneumonia in his original description of ARDS (because, in his view, viral pneumonia looked like RDS of the newborn). COVID exposed the pitfall of oversimplification and lumping over decades disparate diseases by guessing a set of non specific thresholds to capture the diseases for RCT. (so called “pathological consensus”)
Of course COVID pneumonia was not the only outlier disease, it was simply the most overt as an outlier and it was so plentiful that it’s outlier status could not be averaged away by broad application of a protocol. Now it was clear that lumping all these diseases with disparate pulmonary pathology and pathophysiology for protocols and for RCT clearly was (in retrospect) a mistake. However, even after the pandemic, researchers despite 56 years of failure, could not give up on Petty’s idea.
However, after the pandemic, the casual ideas for salvaging Petty’s ideas became very complex and layered as researcher clung to Petty’s original 1960s idea. Maybe, some experts mused, they could just exclude severe COVID pneumonia as an anomaly rather than recognize it as the counter instance it was. Some experts even proposed calling the rest of ARDS, “non-COVID ARDS”.
The latest ESICM ideas for unified ARDS trials are so complex and fluidic that RCT applying the 1960s lumping dogma do not appear to rise to the statistical level (see previous post for citation). Whether or not severe COVID pneumonia is still included in ESICM 2023 “ARDS” is not clear. After all, how could it be?
Yet, in the 1960s an alternative approach to parsimony was bypassed by Dr. Petty. This is the tried and true simple approach of studying each disease individually. (eg RDS of the newborn, RDS of COVID pneumonia, RDS of Influenza A, RDS of a given drug, RDS of inhaled vomit, RDS of post trauma, or RDS of pancreatitis.)
This is traditional parsimony wherein the measurements relate to detection of an objective disease and the “thresholds” only define severity, not the guessed “synthetic syndrome” itself. This approach is universal in much of medicine and profoundly simpler but it requires much more administrative complexity with advanced multicenter research to achieve high enough cases for each trial. That’s something Petty could not do in the 60s (so he had to lump) but such trials are quite possible now. The reluctance to abandon the ARDS (unified syndrome) as an RCT platform is understandable.
Perhaps a common pathway will be found.
However, such trials must be performed within the statistical domain. The RCT measurements must rise to the statistical level. If this is not possible, then performing RCT directed to respiratory failure associated with each specific disease (and not ARDS as a unified state) must be considered and the pitfalls of the dogma must be taught and promulgated and not hidden within the complexity of evolving attempts to salvage Dr. Petty’s original 1960s idea.
lex parsimoniae