I am neither a MD nor a statistician but it seems to me that their procedures introduce some bias. To give the authors benefit of the doubt, if there’s not a (serious) bias then I think that the paper’s title (as well as the Background and Conclusions sections of the Abstract) is too general as it implies that the study results are applicable to all “patients who had heart failure with a reduced ejection fraction”. (Of course, authors must contend with word/character limits)
For example, wouldn’t the initial run-in phases alter either the patients’ physiology or disease progression in ways that wouldn’t mimic a “new” patient? That is, would the outcome be different if the patient first presented with heart failure (HF), never having taken any ACE inhibitor or related drugs? Or whenever a doctor would first prescribe such a medication for treatment of HF. I only glanced at a few replies to the study but it looks like one letter to the Editor expressed a similar point to this.
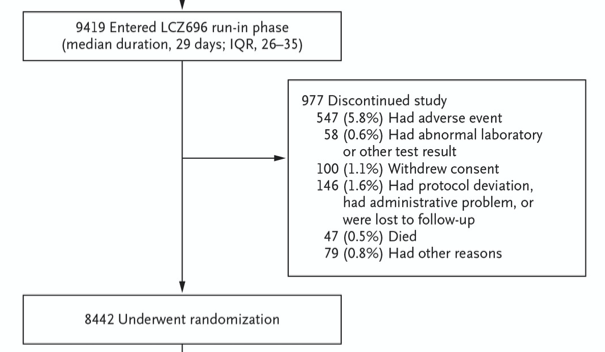
They also had an initial run-in phase of 2-3 weeks in which participants received enalapril (10 mg twice daily) prior to the run-in phase with LCZ696. N = 1,102 patients discontinued the study at this phase (N = 591 for having an adverse event). However, in the Introduction the authors state that
The effect of angiotensin-receptor blockers (ARBs) on mortality has been inconsistent,3,4 and thus, these drugs are recommended primarily for patients who have unacceptable side effects (primarily cough) while receiving ACE inhibitors.
So shouldn’t they want to assess the effect of the new drug in these patients (i.e., those N = 591 who were excluded due to adverse side effects from enalapril), in addition to those who underwent randomization?
They “bury” the following in the second-to-last paragraph of the paper (the paragraph which is worded like a Limitations section without calling it one, which I personally dislike):
During enrollment, we evaluated patients who were already taking various doses of ACE inhibitors or ARBs and required that they be able to take the equivalent of a relatively low dose of enalapril (10 mg daily) without unacceptable side effects.
But if LCZ696 is intended to replace enalapril (or other ACE inhibitors), then “does it matter” that taking enalapril would result in adverse side effects?