Here’s a link to a press item describing a RCT to compare the safety of R-CHOP plus tafasitamab (MOR208) to R-CHOP plus tafasitamab and lenalidomide (Revlimid) in treatment-naive DLBCL
http://bit.ly/2RFkZJx
Treatment -naive DLBCL is cured ~60% of the time with backbone R-CHOP in previously untreated patients with this aggressive lymphoma - which is less likely to be cured second line.
Red flag: This is a safety study in a first line treatment setting.
Ethical concern:
Is it ethical to compare 2 experimental approaches in a curative setting? 1 or both study combinations may lead to unanticipated AEs leading to decreased efficacy relative to SOC: R-CHOP.
Efficiency concern:
the study teams may need to expose more patients to experimental treatments over a longer period of time to know which should to bring forward in an RCT against R-CHOP (compared to studying each combination against R-CHOP from the start.)
I feel this should be the way forward - do 2 studies up front:
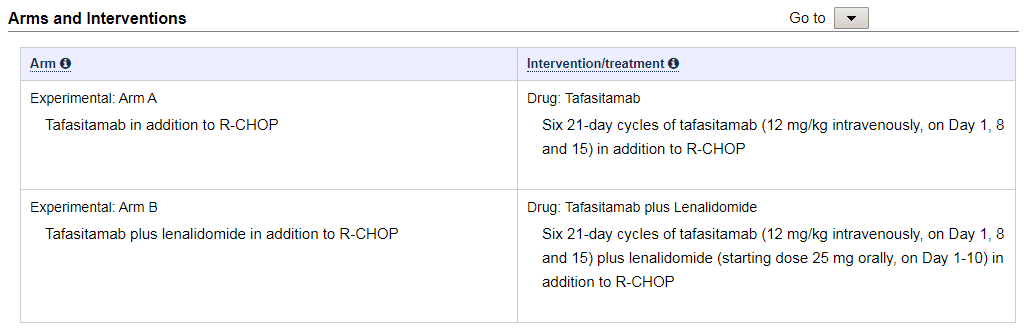
R-CHOP plus tafasitamab VS R-CHOP (half get SOC)
AND:
R-CHOP plus tafasitamab and lenalidomide VS R-CHOP (half get SOC)
The above comparisons provide a reference point in each study for judging safety and signals for efficacy relative to the SOC. As done, both may be less, or as, safe as R-CHOP and we would not know.
Endpoints copied from study enrolling 60 patients: https://clinicaltrials.gov/ct2/show/NCT04134936
Primary Outcome Measures :
- Incidence and severity of treatment-emergent adverse events (TEAEs) [ Time Frame: 6 months approximately ]
Secondary Outcome Measures :
-
Objective Response Rate (ORR) at the end of treatment [ Time Frame: 6 months approximately ]
-
Metabolic, PET-negative complete response (CR) rate at the end of treatment [ Time Frame: 6 months approximately ]
-
Incidence and severity of adverse events (AEs) in the follow-up period [ Time Frame: 18 months approximately ]
-
Best Objective Response Rate (ORR) until the end of study [ Time Frame: 24 months approximately ]
-
Metabolic, PET-negative complete response (CR) rate until the end of study [ Time Frame: 24 months approximately ]
-
Progression-free survival (PFS) at 12 and 24 months [ Time Frame: 24 months approximately ]
-
Event-free survival (EFS) at 12 and 24 months [ Time Frame: 24 months approximately ]
-
Time to next anti-lymphoma treatment (TTNT) [ Time Frame: 24 months approximately ]
-
Overall survival at 12 and 24 months [ Time Frame: 24 months approximately ]
-
Anti-tafasitamab antibodies formation [ Time Frame: 12 months approximately ]
Jan 21 addition:
In this safety study there is no titration of the study drugs or dose level groups, 1 or both study drugs are given concurrently with the SOC at the active dose level (determine in other studies).
Thoughts?
About me: Karl Schwartz, formerly: patient representative to FDA, president of PAL, CIRB member, NCI Steering Cmt, Vail Methods Workshop faculty. www.lymphomation.org
2 Likes
So long as the participants get full dose and cycles of R-CHOP that they have received standard of care in respect to potential efficacy – so in this circumstance I think going step-wise with dosing is prudent. Starting with R-CHOP+ taf in a single arm study. A safety study to find the right dose.
Once completed they can be more confident about safety of dose for combos in a RCT with R-CHOP as the control to hopefully improve on the SOC.
The combination can improve efficacy (hopefully, but maybe not) but can also lead to SAEs that may delay receiving all of the R-CHOP – or worse – even if not anticipated.
Dose deescalation after cycle 1 will not undo deaths that we cannot assume will not result from receiving untested full dose of 1 or 2 study drugs with R-CHOP in the planned study. Tumor lysis for example.
1 Like
Adapting @davidcnorrismd individualized step-up model, asking why not:
Cycle 1:
1/4 dose of study drug(s) given with R-CHOP (or some other fraction)
Cycle 2-6: if no SAE individual step-up/down doses by some fraction.
Consider extended dosing of study drugs for additional 1-2 cycles
Endpoints:
-Portion of patients who can receive full dose of study drug(s) without dose reductions of standard curative R-CHOP
-Starting dose and range of doses for extension of study
to phase III testing efficacy against R-CHOP
- Correlates - blood markers associated with Serious AEs in study arm.
No pretense that that study as planed is a valid RCT design.
The control to assess toxicity must also be the standard of care.
Know what we don’t know: there is no certainty that full dose of study drug(s) will not cause serious AEs including death. Which is unacceptable for an indication that is curable at about 60%.
1 Like
Karl, thank you for highlighting this interesting example. My sense of “safety study” is that this language is used pro forma here, and may not necessarily reflect the full underlying rationale for the study.
As you note, first-line treatment is an opportunity not to be wasted. Interestingly, a paper that recently came to my attention [1] actually begins from that same premise to challenge the very concept of standard of care (SOC) that you invoke as the basis for your challenge to this trial!
From a perspective more aligned to this forum, I think what one might like to see by way of justification of the First-MIND trial (NCT04134936) is a clinical prediction model showing that the patients with “intermediate- to high-risk disease” enrolled have a risk specifically targeted by the anti-CD19 tafasitamab. One could even imagine conducting the trial in an adaptive, learn-as-you-go spirit such that the highest-risk patients enrolled first, since they would be the ones for whom SOC is least attractive and for whom the intervention might be most impactful.
- Subbiah V, Kurzrock R. Challenging Standard-of-Care Paradigms in the Precision Oncology Era. Trends Cancer. 2018;4(2):101-109. doi:10.1016/j.trecan.2017.12.004 PMC5822744
2 Likes
Appreciate the comments, David. (Apologize for the delay in seeing your response.)
Agree that safety is a pro forma description.
My understanding is that cd19 is universally expressed on DLBCL b-cell tumors.
Agree that selecting participants with high risk DLBCL would mitigate the concerns I’ve raised – such as so-called double-hit DLBCL which has a lower cure rate. Apparently, this was not part of eligibility:
1 Like