“…and the OR is portable across baseline prevalence of an outcome…” As far as I can see, that statement is a non-sequitur and foregone conclusion that recurs throughout your posts and papers on this topic, along with other logical fallacies and easily refutable assertions. I’ll try to summarize the major ones:
The matter of portability is in the end empirical. Anyone can look at real study reports to see how measures vary across studies. Provided that one does not commit the nullistic fallacy of confusing p>0.05 with absence of variation, it is easy to see that all measures usually vary quite a bit across baseline risks, which is often just as expected from knowledge of the underlying causal mechanisms. That the OR might vary less could just as well be taken as a defect of the OR for some purposes, insofar as baseline risks are of crucial practical importance in many if not most real applications.
To assert empirical claims like “…and the OR is portable across baseline prevalence of an outcome…” based on math results is an example of reification of abstractions that at best are theories or hypotheses that need to be evaluated against data. Empirical studies have been done by different parties and some have noted how others commit further fallacies by declaring greater portability for ORs based on statistics that are incommensurable across measures.
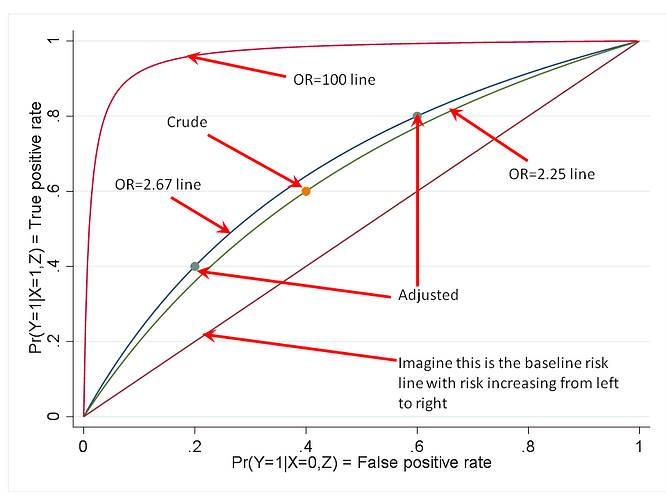
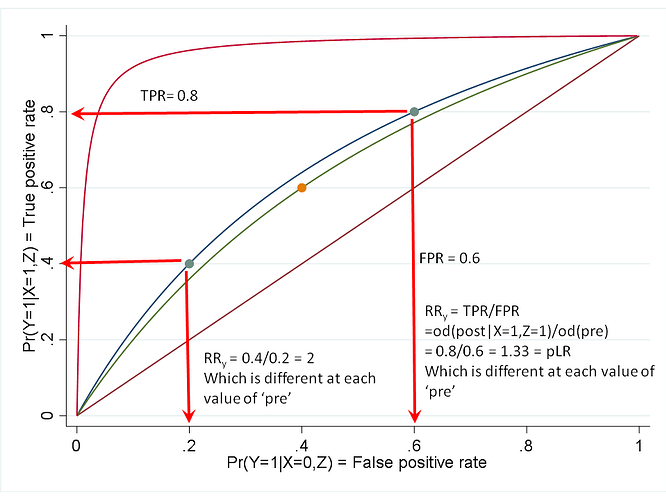
The ultimate fallacy in the quoted claim is however the degradation of a defensible (albeit still empirical) thesis, “by some criteria, the OR tends to be more portable than the RR”, into the flat assertion that “…the OR is portable across baseline prevalence of an outcome…”, which is simply false: Put random positive numbers that sum to 1 into a series of 2x2 tables to create a hypothetical set of expected counts, and you will almost surely see variation across the tables in both baseline prevalence and every measure, including the OR.
As usual, my practical views aren’t very far from Frank’s, experience being a potent source of convergence, and I have already explained them at length in this thread and others over the years here on Datamethods. First, variation dependence among parameters can contribute to heterogeneity via range restrictions. Risk ratios have that heterogeneity source when the outcome becomes common enough in some comparison groupings; the source is even stronger for risk differences. In contrast, odds ratios and hazard rate ratios do not have that mathematical source of heterogeneity. But there are many real-world sources of heterogeneity that affect all measures; most of them are uncontrolled in real study reports and usually unmeasured in real data sets. Thus there is no compelling case for ignoring heterogeneity in any measure, other than simple inability to estimate it reliably with available data.
The ubiquity of heterogeneity as well as cost-benefit considerations show that we need to focus on estimating practice-relevant features of entire conditional distributions of multivariate outcomes, at least if we are concerned with addressing medical problems rather than math problems. Every summary measure is deficient and potentially misleading for practical purposes because it ignores or discards important information. You shouldn’t buy a car based on its horsepower alone, nor should you buy a cancer treatment based on a summary measure of 5-year survival alone - after all what if it extends your life 4 years, but those 4 years are as locked-in paralysis?
For all these reasons and others I have discussed here over what is now years, I find your obsession with promoting ORs misguided at best, and misleading if taken seriously. Where we may agree is this: Suppose one knows nothing about the causal mechanisms to suggest a particular model and (as always) the data are too limited to approach the above-stated ideal. Then, for statistical stability and simplicity reasons, it makes sense to use a model form linear in the natural parameter of the data distribution (logistic for risks, loglinear for counts and hazard rates, etc.) that obeys the logical range restrictions of the outcome. This choice makes the usual large-sample approximations (e.g., Wald P-values and CIs) most accurate in finite samples, and in the logistic case makes ORs the easiest summary measures to calculate. What it emphatically does not do is make the OR the most relevant measure in any contextual, scientific, policy or medical sense. For those real-world purposes the ideal target is again the entire conditional multivariate distribution of the outcomes of concern, and any summary measure can lead to distorted inferences and bad decisions due to overlooking the information the summary disregards.